

Misrepresent that a product or activity is infringing your copyrights. Please be advised that you will be liable for damages (including costs and attorneys’ fees) if you materially Your Infringement Notice may be forwarded to the party that made the content available or to third parties such Means of the most recent email address, if any, provided by such party to Varsity Tutors. Infringement Notice, it will make a good faith attempt to contact the party that made such content available by If Varsity Tutors takes action in response to Information described below to the designated agent listed below. Or more of your copyrights, please notify us by providing a written notice (“Infringement Notice”) containing If you believe that content available by means of the Website (as defined in our Terms of Service) infringes one Our final order is, or II < III < IV < I < V. The strongest form of intermolecular force is ionic forces, which exist in ionic compounds like.

experiences hydrogen bonding because it has hydrogen atoms bonded to oxygen atoms. Because hydrogen bonds are a stronger form of dipole interactions, this puts next in the list. Next is hydrogen bonding, an especially powerful form of dipole interactions when hydrogen is bonded to a fluorine, oxygen, or nitrogen atom. is a polar molecule and experiences dipole interactions, which makes it the next strongest in our list. The next tier of IMF is permanent dipole interactions (dipole-dipole interactions) that are not hydrogen bonds. Therefore, is weaker, because it has less electrons available than. In general, the more electrons that are available to push, the more potential there is for a dipole to occur. Non-polar molecules like and can still exhibit temporary dipoles by induction, when the electrons of one molecule push away the electrons of another. Since the question asks us to order the compounds from least strength to greatest, we'll start with the weakest IMF: Van der Waals forces, also called "induced dipoles" or London dispersion forces. There are four broad categories of IMFs, all of which are represented here. This is a question about intermolecular forces, or IMFs. The hydrogen takes on a partial positive charge and the electronegative atoms takes on a partial negative charge. The strongest intermolecular force is hydrogen bonding, which is a particular subset of dipole-dipole interactions that occur when a hydrogen is in close proximity (bound to) a highly electronegative element (namely oxygen, nitrogen, or fluorine). Intermolecular forces will never change the identity of the molecule and cannot be used to add atoms to a compound. Hydrogen bonds, dipole-dipole interactions, and van der Waals forces (London dispersion forces) are some common examples of intermolecular forces. These attractions are constantly broken and reformed as molecules move around.
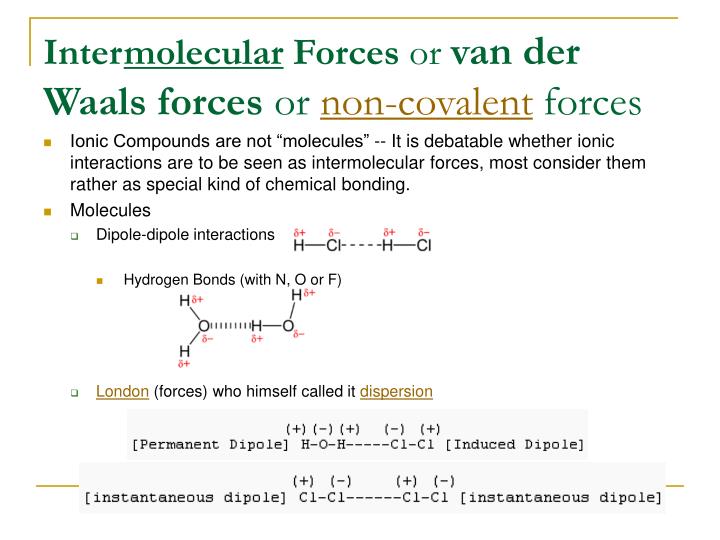
Intermolecular forces, in contrast, are more transient and less stable. An atomic bond will change the identity of a compound by adding an atom to the structure. This means that they are generally stable and relatively irreversible. Ionic bonds and covalent bonds are atomic bonds, meaning they are intramolecular. There is a key difference between atomic bonds and intermolecular forces.


 0 kommentar(er)
0 kommentar(er)
